
The consequence of rear-side bonding by the nucleophile is an inversion of configuration about the alpha-carbon.
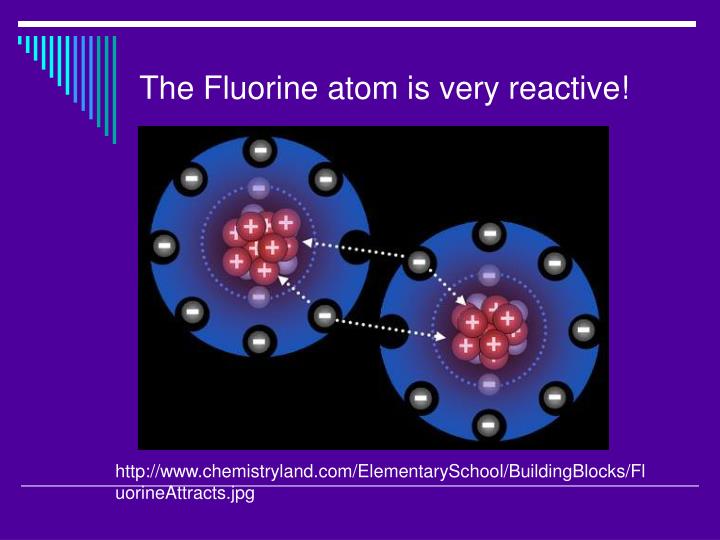
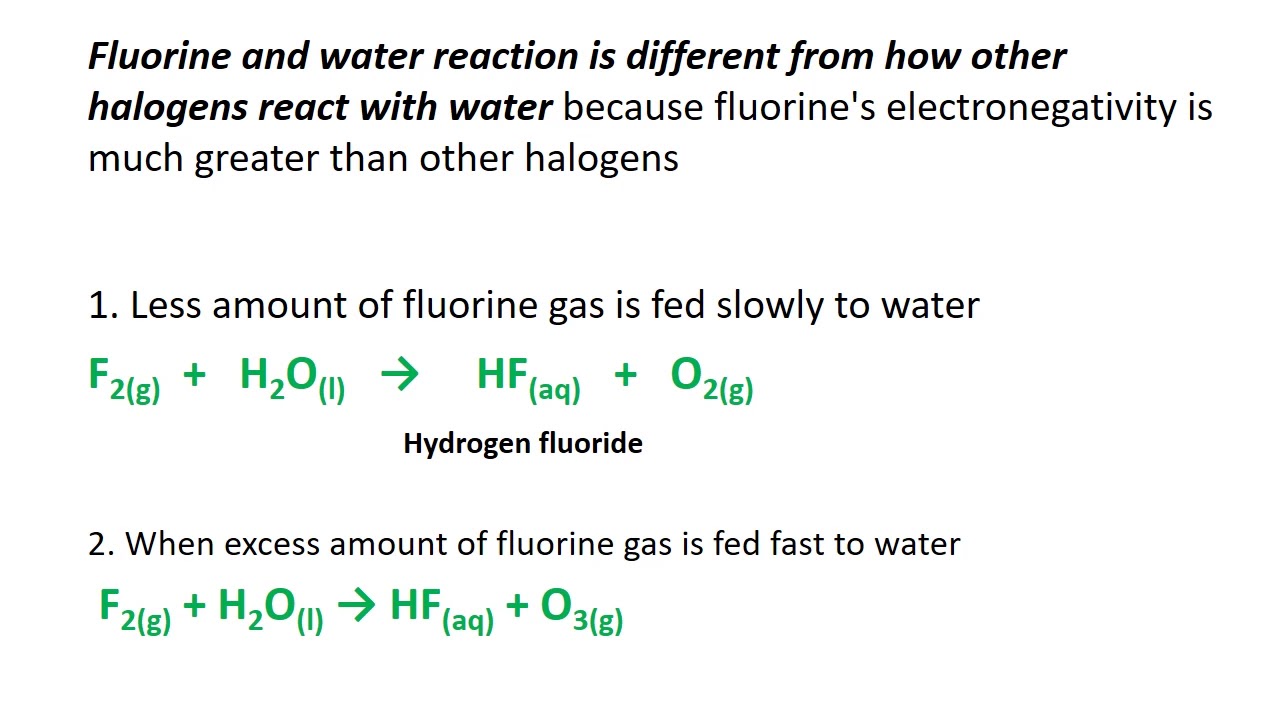
#FLUORINE REACTIVITY FULL#
Both the nucleophile and the halogen bear a partial negative charge, the full charge being transferred to the halogen in the products. In the S N2 transition state the alpha-carbon is hybridized sp 2 with the partial bonds to the nucleophile and the halogen having largely p-character. We call this description the S N2 mechanism, where S stands for Substitution, N stands for Nucleophilic and 2 stands for bimolecular (defined below). The diagram on the right shows this process for an anionic nucleophile. As a covalent bond begins to form between the nucleophile and the carbon, the carbon halogen bond weakens and stretches, the halogen atom eventually leaving as an anion. The nucleophile must approach the electrophilic alpha-carbon atom from the side opposite the halogen. We can now piece together a plausible picture of how nucleophilic substitution reactions of 1º and 2º-alkyl halides take place. With the exception of HF (pK a = 3.2), all the hydrohalic acids are very strong, small differences being in the direction HCl ( R)-CH 3 CH N 3CH 2CH 3 + Na I This stability may be estimated from the relative acidities of the H-X acids, assuming that the strongest acid releases the most stable conjugate base (halide anion). The second factor to be considered is the relative stability of the corresponding halide anions, which is likely the form in which these electronegative atoms will be replaced.
The carbon-chlorine covalent bond is slightly weaker than a carbon-carbon bond, and the bonds to the other halogens are weaker still, the bond to iodine being about 33% weaker. Because of this, alkyl fluorides and fluorocarbons in general are chemically and thermodynamically quite stable, and do not share any of the reactivity patterns shown by the other alkyl halides. Remarkably, this is the strongest common single bond to carbon, being roughly 30 kcal/mole stronger than a carbon-carbon bond and about 15 kcal/mole stronger than a carbon-hydrogen bond. The strongest of the carbon-halogen covalent bonds is that to fluorine. The first of these is covalent bond strength. Two characteristics other than electronegativity also have an important influence on the chemical behavior of these compounds. Consequently, this functional group is polarized so that the carbon is electrophilic and the halogen is nucleophilic, as shown in the drawing on the right. With the exception of iodine, these halogens have electronegativities significantly greater than carbon. The functional group of alkyl halides is a carbon-halogen bond, the common halogens being fluorine, chlorine, bromine and iodine. CCl 2F 2 and other chlorofluorocarbons) used as refrigeration gases and fire extinguishing agents. Other organic halogen compounds that have been implicated in environmental damage include the polychloro- and polybromo-biphenyls (PCBs and PBBs), used as heat transfer fluids and fire retardants and freons (e.g. 2,4,5-T and 2,4-D are common herbicides that are sold by most garden stores. Because DDT is a cheap and effective mosquito control agent, underdeveloped countries in Africa and Latin America have experienced a dramatic increase in malaria deaths following its removal, and arguments are made for returning it to limited use. have been used as pesticides, but their persistence in the environment, once applied, has led to restrictions, including banning, of their use in developed countries. Some halogen compounds, shown in the box. Many of these have proven useful as intermediates in traditional synthetic processes. Synthetic organic halogen compounds are readily available by direct halogenation of hydrocarbons and by addition reactions to alkenes and alkynes. Many subsequent chemical and biological processes produce poly-halogenated methanes.
Furthermore, the ocean is also estimated to supply 10-20% of atmospheric methyl chloride, with other significant contributions coming from biomass burning, salt marshes and wood-rotting fungi. The ocean is the largest known source for atmospheric methyl bromide and methyl iodide.

However, the halogen rich environment of the ocean has produced many interesting natural products incorporating large amounts of halogen. The thyroid hormones T 3 and T 4 are exceptions as is fluoroacetate, the toxic agent in the South African shrub Dichapetalum cymosum, known as "gifblaar". Halogen containing organic compounds are relatively rare in terrestrial plants and animals.


 0 kommentar(er)
0 kommentar(er)
